Alloplex's SUPLEXA therapy and the SUPLEXA-101 in-human trial in Australia.
SUPLEXA is a cellular therapy made specifically for each patient from their own blood. Blood is drawn in the usual manner and sent to a central manufacturing facility where a laboratory process is used to activate the white blood cells to recognize and kill cancer cells.
These tumor-killing cells, called SUPLEXA cells, are stored frozen until requested by the treating physician. The frozen SUPLEXA cells are prepared for intravenous administration (IV).
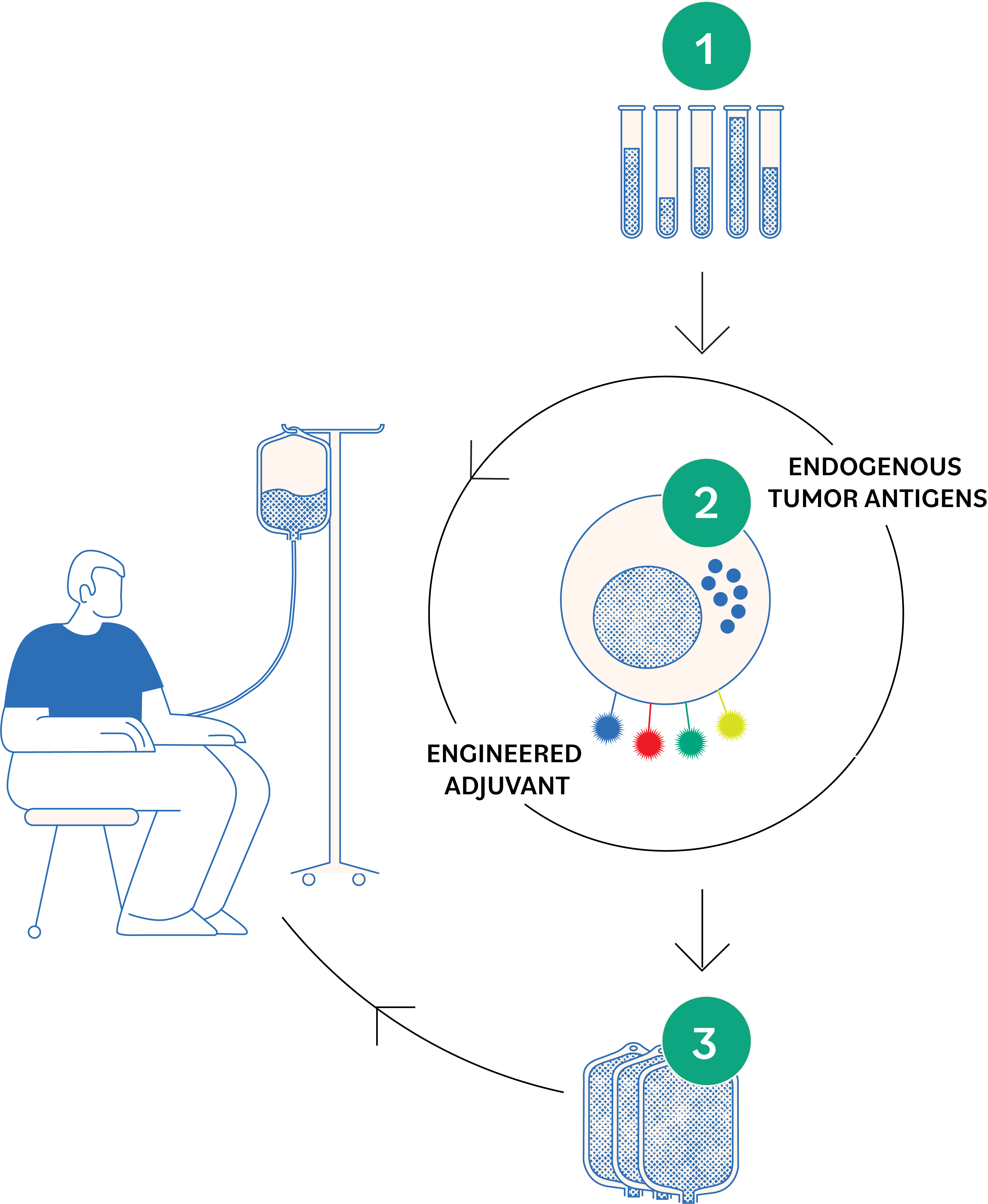
Small volume of whole blood is drawn from the patient and shipped overnight to a regional laboratory to isolate the white blood cells.
White blood cells are co-incubated with ENLIST training cells to induce the production of SUPLEXA therapeutic cells. SUPLEXA cells are expanded to high numbers.
Patient receives a number of doses of SUPLEXA therapeutic cells using a standard intravenous line.
SUPLEXA therapeutic cells - which are made of non-engineered, normal activated immune cells - use many different tumor-killing mechanisms to attack the cancer.
Early laboratory studies with SUPLEXA cells show they are very potent against a variety of tumor cells, while having no effect against normal healthy cells.
Results of the laboratory research suggested a highly-favorable safety and tolerability profile. In January 2022, Australia's Human Research Ethics Committee 'HREC' approved Alloplex's submission to proceed to in-human trials. In April 2022, Alloplex Biotherapeutics announced the site opening for its first-in-human (FIH) clinical trial of SUPLEXA therapeutic cells.
Recent clinical experience shows that SUPLEXA cells are also well tolerated in cancer patients.
Phase 1 'SUPLEXA-101' trial (NCT05237206) was conducted in Australia across three sites: at Cancer Research SA (CRSA) with Assoc. Professor Rohit Joshi as the Principal Investigator at St Andrew's Medical Centre; with Dr. Ganessan Kichenadasse at Southern Oncology Clinical Research Unit (SOCRU) both in Adelaide, South Australia; as well as with Dr. Jeff Goh at Gallipoli Research Medical Foundation, Greenslopes Hospital in Brisbane, Queensland. It concluded in June 2024.
For this initial trial, subjects needed to meet defined entry criteria and have measurable relapsed or refractory advanced malignancy for which no standard therapy exists. The primary outcome measure of the trial was to assess safety and tolerability of SUPLEXA in subjects with malignant solid tumors and haematologic malignancies.
The final results of this trial will be presented at the annual meeting of the Society for the Immunotherapy of Cancer (SITC) to be held in Houston, Texas, USA in November 2024.
Patients interested in the ongoing SUPLEXA clinical trial can find details for the trial sites here: